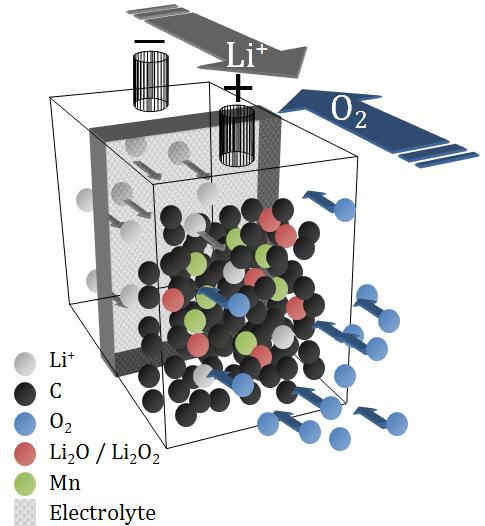
There is no other battery that has a potential to provide up to 10 times energy density like the Lithium Air. In a Lithium Air battery, the anode is made of lithium. The cathode is oxygen, drawn from the surrounding air. Instead of carrying Oxygen as a reagent in the battery as in the Lithium Ion battery, the Lithium Air battery use oxygen available abundantly from the environment, thus, we can save weight and volume.
As the lithium oxidizes, it releases energy. Pumping electricity into the device reverses the process, expelling the oxygen and leaving pure lithium. While it is currently feasible to have a lithium-air battery for one-time usage, it is a great challenge to make Lithium Air rechargeable.
The technology requires significant research in a variety of fields before a viable commercial implementation is expected. In the last ten years, many materials for lithium–air battery systems have been reported by many researchers; especially non-aqueous electrolytes, water-stable solid electrolytes, and catalysts for Li2O2 reduction.
